Introducing Xdemvy: A Breakthrough in Demodex Treatment
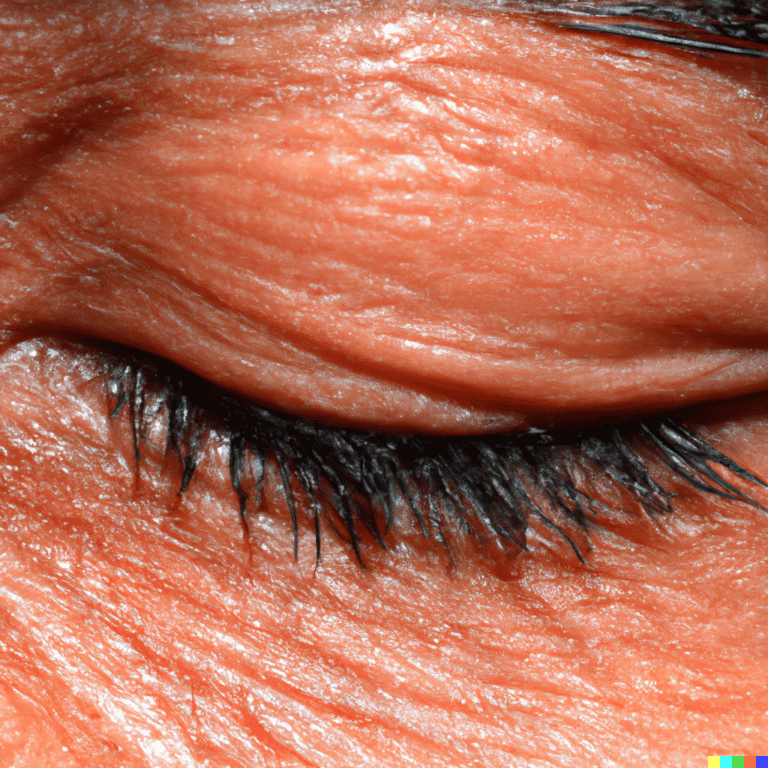
Are you struggling with red, itchy eyes? Have you been diagnosed with Demodex blepharitis? If so, there’s exciting news for you! The FDA has recently approved a new drug called Xdemvy, a revolutionary treatment for Demodex blepharitis. Let’s explore what this means for you.
What is Demodex Blepharitis?
Demodex blepharitis is a common eyelid disease caused by an infestation of Demodex mites. These mites can lead to symptoms like redness, inflammation, missing or misdirected eyelashes, and the presence of collarettes (cylindrical, waxy debris) at the base of the eyelashes.
Xdemvy: The First FDA-Approved Treatment for Demodex
Xdemvy (lotilaner ophthalmic solution 0.25%) is the first and only FDA-approved treatment that directly targets Demodex mites, the root cause of Demodex blepharitis. This new medicine offers a positive step forward for the treatment of this disease, providing relief for many patients who have been struggling for years.
How Does Xdemvy Work?
Xdemvy (lotilaner ophthalmic solution 0.25%) is designed to directly target Demodex mites, the root cause of the condition. Patients with Demodex blepharitis are prescribed Xdemvy to be dosed twice daily in each eye over a 6-week course. The drug’s efficacy has been demonstrated through significant improvements in the condition of the eyelids, including the reduction of collarettes, a key sign of the disease. Additionally, the endpoints of mite eradication and erythema cure showed statistically significant improvement across clinical studies. Some patients even reported seeing improvements as early as 2 weeks into the treatment. Xdemvy’s targeted approach offers a promising solution for those suffering from Demodex blepharitis, addressing the underlying cause of the disease rather than merely treating the symptoms.
Is Xdemvy Safe?
The safety of Xdemvy, a treatment for Demodex blepharitis, was rigorously evaluated through two comprehensive studies (Saturn-1 and Saturn-2), involving 833 patients. These trials found the drug to be generally safe and well-tolerated. The most common ocular adverse reaction was stinging and burning at the instillation site, reported in 10% of patients, while less common reactions like chalazion/hordeolum (stye) and punctate keratitis were reported in less than 2%. These side effects are considered minor, and the long-term safety profile of Xdemvy will continue to be monitored to identify any rare or delayed adverse effects as more patients use the drug.
Cost and Availability
Xdemvy will cost $1,850 per prescription, expected to last about a year. Tarsus, the company behind Xdemvy, plans to launch an assistance program to keep the cost to patients at $100 or less. Commercial provider coverage is expected in 2024, with Part D Medicare coverage in 2025.
Natural Remedies for Demodex
While some individuals may explore natural remedies for Demodex, such as tea tree oil, aloe vera, coconut oil, lavender oil, and dietary changes, it’s important to approach these with caution. These remedies are often cited for their antiseptic, anti-inflammatory, or antimicrobial properties, but their effectiveness in treating Demodex mites is not well-established. More importantly, at Compton Eye Associates, we do not recommend these therapies. They can cause irritation or other adverse reactions, especially around the sensitive eye area. It’s essential to consult with eye care professionals to understand the best and safest course of treatment for Demodex, rather than relying on unproven natural remedies.
Schedule an Appointment with Compton Eye Associates
At Compton Eye Associates, we are committed to providing the best eye care and Demodex treatment options, including the newly approved Xdemvy. Don’t let Demodex blepharitis impact your life any longer. Schedule an appointment with our expert doctors today and take the first step towards clear, comfortable eyes.
Click here to schedule your appointment with Compton Eye Associates